Investor Relations
Overview


A kinder way to immunize against respiratory and enteric infectious diseases. QYNDR is the future vaccination technology.
- Conduct and complete Phase II/III clinical development – COVID-19 vaccine
- Produce cGMP quality product at pre-commercial scale
- Prepare submissions for regulatory approvals
- Advance additional vaccine candidates through IND and up to First-in-Humans Clinical Trials – Influenza, Group A Strep, Pertussis, RSV, others TBD
QYNDR: Use of Funds and Head Count Projections
Business Development & Sales
Governments purchase vaccines. Requires specialized government contracting and business development people.
Clinical Trial Execution & Global Regulatory Filings and Registrations
Contracting and managing contract research organizations (CRO) globally who conduct the clinical trial. This includes data collection, external analytical laboratory management, and regulatory filings.
CMO / Supply Chain Setup
Staff must develop and qualify supply chain with CMO to support clinical materials as they cannot be changed after application.
Clinical Materials Procurement
Staff trained and experienced with contract manufacturing will build processing lines capable of supporting clinical trials and full BLA approval for the oral vaccines.
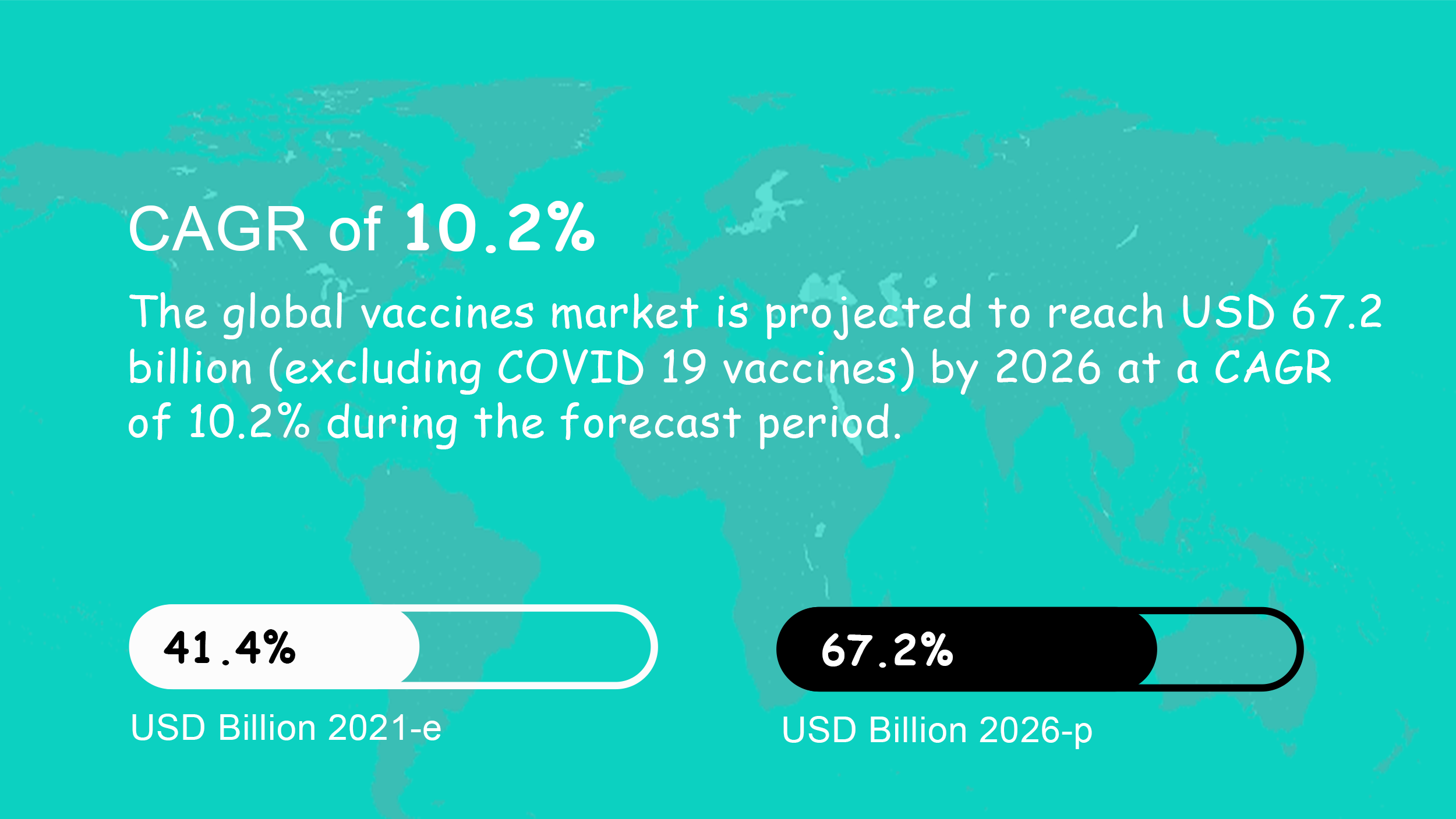
Global Market: $41.4 Billion / CAGR 10.2%*
- Global Vaccine Market projected to reach $149.2 billion by 2026 (Including COVID-19). The international community needs better vaccination platforms and more of them.
- It is in the strategic interest of the United States to ensure that vaccines are available for citizens and visitors alike are. This oral platform is able to address global vaccination needs for respiratory and enteric infectious disease now and in the future.
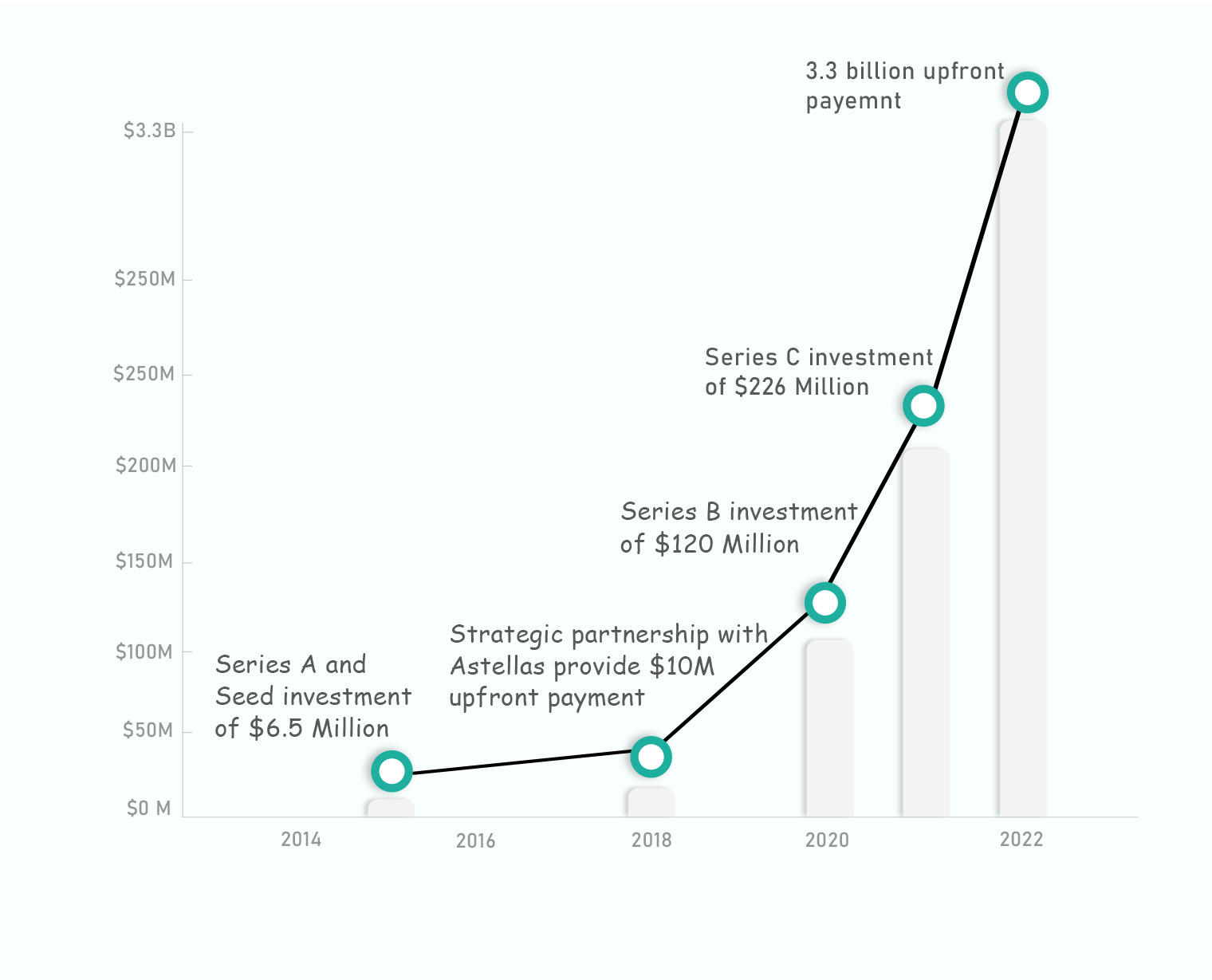
Comparison with Precedent Transaction: 9 – 14X Return in 15 Months to Series C Investors
Example of a recent M&A action from a large Pharma Company
- Proposed acquisition provides access to next-generation 24-valent pneumococcal vaccine candidate in phase II development and highly innovative. MAPStm technology
- Supports development of a strong portfolio vaccines and specialty medicines
Quick Links
Other Pages
Contact Info
- 17 S. Commerce Way #20747, Lehigh Valley, PA 18002-0747
- +1 610 585 9536
- info@ussfgmp.com
Copyright © 2023. All rights reserved.
