Pipeline
Projects Under Development
Drs Flanigan and Morefield lead their development and manufacturing teams to commercialize QYNDR, a oral mucosal vaccine delivery platform against several different disease targets. Any vaccine commercialized will be easier to use, have a longer shelf-life and may provide protection for longer with fewer side effects. The following table provides our pipeline of projects currently under development:
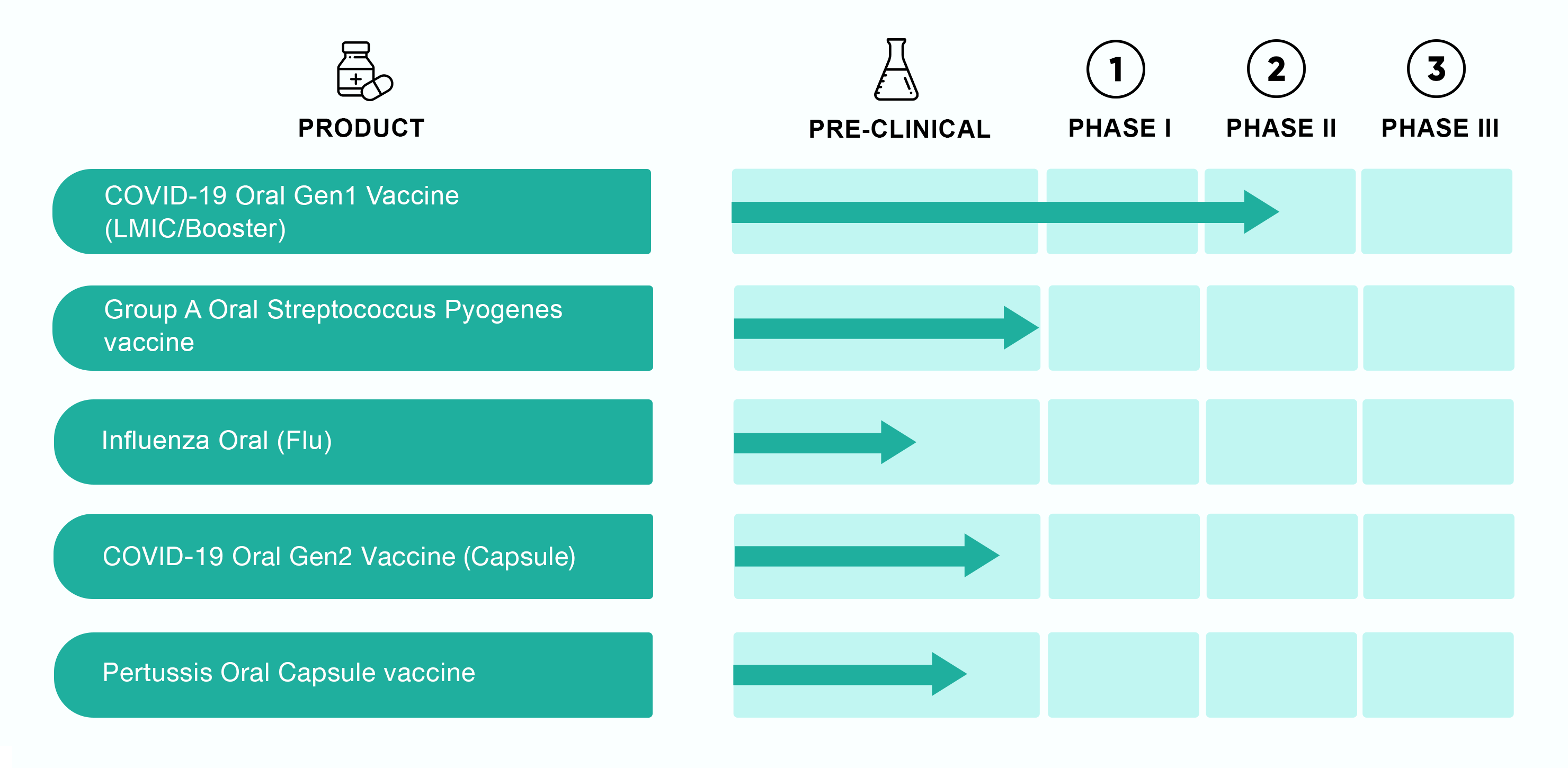
Please contact USSF (info@ussfgmp.com) for the latest information regarding clinical trials and our development roadmap programs.
Covid-19 & Flu are endemic with vaccination recommended each year.

A. Human Clinical Trial Phase I successfully completed, no safety issues and excellent “break-through” performance.
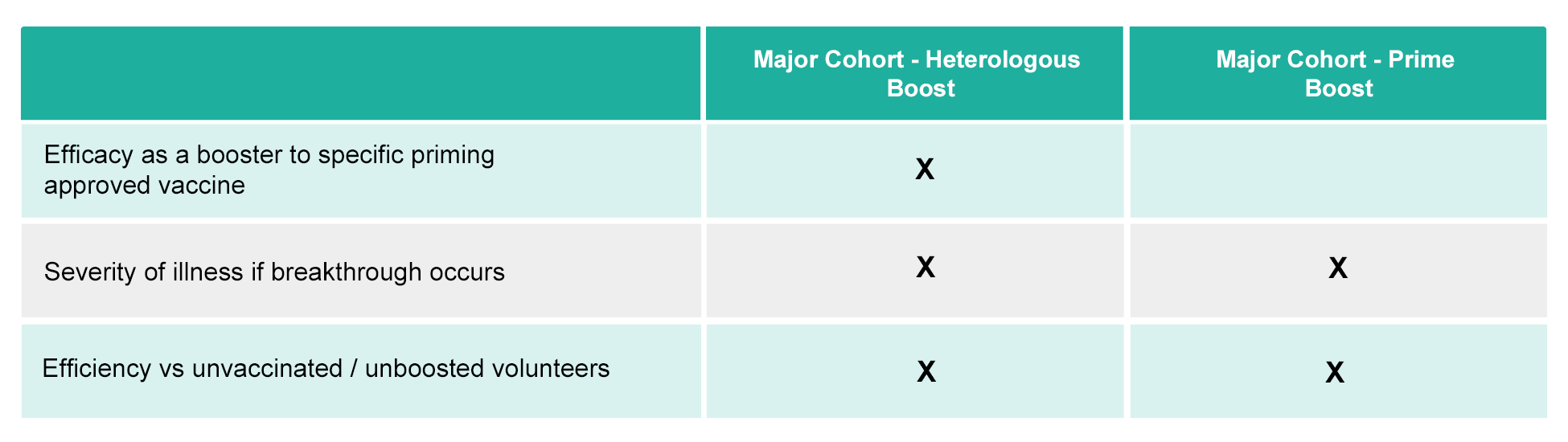
B. Oral Vaccine Human clinical gated Phase II/III trials are designed with endpoints measuring the following.
- Major Cohort – Heterologous Boost: These cohorts will be targeted in populations with access to previously approved vaccines
- Major Cohort – Prime and Boost: These cohorts will be targeted in populations with access to previously approved vaccines
C. Clinical Trial Design
- Decentralized clinical trial methodologies will be used w/a 2x lab facilities handling serum and mucosal specimen testing
- Diversity, Equity and Inclusion (DEI) based site selection is critical in ensuring a robust participant pool. This is key as global immunization is of strategic importance with the USA as a hub of commerce and innovation
